
Launching our new Feature at the ASHP Vegas Show: Pharmacy Marketplace Integration
The TRXADE Marketplace is one of the most trusted marketplace for pharmacies and wholesalers with over 6000 members.
Home » Traceability » Page 9

The TRXADE Marketplace is one of the most trusted marketplace for pharmacies and wholesalers with over 6000 members.

The Drug Supply Chain Security Act (DSCSA) requires that starting November 2017 manufacturers mark their products in human and machine-readable format with a National Drug Code (NDC).

Get detailed updates on FDA – HDA Traceability Seminar. Acquaint yourself with the requirements of new pharma laws.

Enacted in 2013, DSCSA is considered a heavy burden by many companies in the pharma supply chain.

As the Drug Supply Chain Security Act deadlines approach, serialization and aggregation coordination efforts come to the fore.

Be aware of the penalties under the Drug Supply Chain Security Act for smooth business and better delivery of pharmaceutical products.
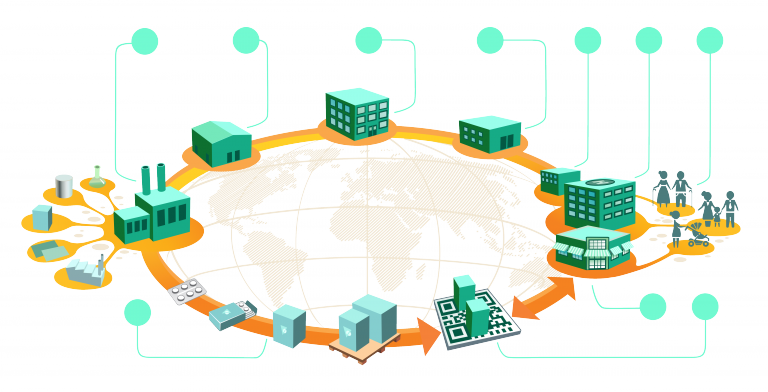
The Importance of Collaboration Across the Supply Chain is important for effectively supplying the products in time by maintaining its quality. Pharma supply chain is not an exception.
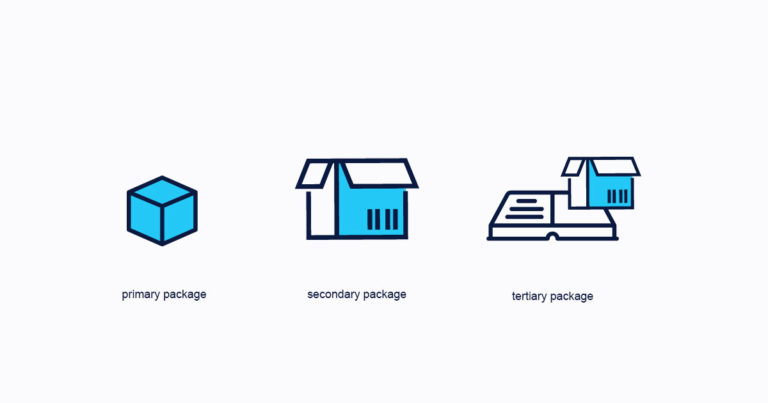
The FDA is finally getting serious about the DSCSA and to prove this they have released a DSCSA workshop to pilot their studies.

Get to know how OPEN-SCS’ New Standards Will Impact Packaging Lines and Serial Number Bonding in the new era of pharmaceutical serialization.
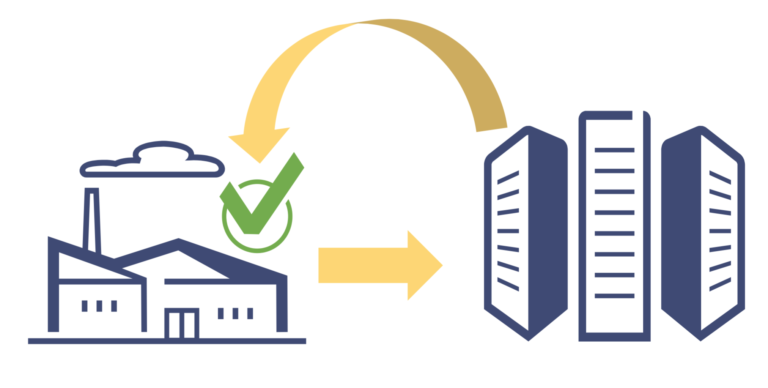
Verification Router Service: How Will It Impact DSCSA Compliance in the pharmaceutical supply chain industry. Know more!
Shaping the future of traceability and serialization since 2007