
HDA Traceability Seminar Recap – FDA Update
Get detailed updates on FDA – HDA Traceability Seminar. Acquaint yourself with the requirements of new pharma laws.

Get detailed updates on FDA – HDA Traceability Seminar. Acquaint yourself with the requirements of new pharma laws.

Enacted in 2013, DSCSA is considered a heavy burden by many companies in the pharma supply chain.

As the Drug Supply Chain Security Act deadlines approach, serialization and aggregation coordination efforts come to the fore.

Be aware of the penalties under the Drug Supply Chain Security Act for smooth business and better delivery of pharmaceutical products.
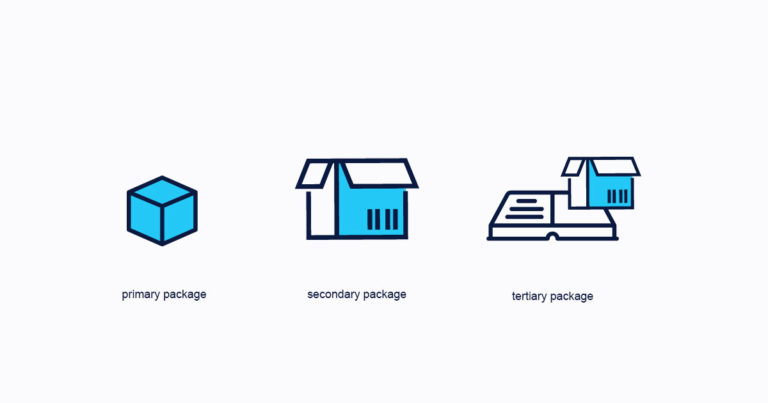
The FDA is finally getting serious about the DSCSA and to prove this they have released a DSCSA workshop to pilot their studies.
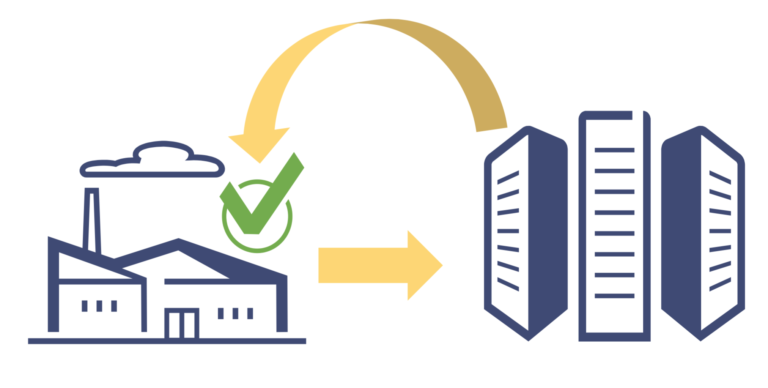
Verification Router Service: How Will It Impact DSCSA Compliance in the pharmaceutical supply chain industry. Know more!
Challenges with Using ASN/EDI for Passing Lot-Based DSCSA Transaction Data. At TrackTraceRx we deal with our clients EDI/ASN integration on a daily basis.

Wholesale Distributor Annual Reporting Deadline. This is one of the most important deadlines in the entire pharmaceutical industry.

A secure web portal with data exchange capabilities is not enough – document storage and retrieval features need to be added to the feature mix for real value addition.

Regarding serialization, there exist a number of questions that need to be answered directly by the FDA. If companies chooses not to randomize, they run the risk of having someone in the supply chain.
Shaping the future of traceability and serialization since 2007