
Comparing the DSCSA and the EU/FMD
The Drug Supply Chain Security Act (DSCSA) mandates the development and implementation of standards for tracing drug products, with these standards to be phased in by 2023.

The Drug Supply Chain Security Act (DSCSA) mandates the development and implementation of standards for tracing drug products, with these standards to be phased in by 2023.

The Trading Partner per DSCSA can provide certain product tracing information in order to become authorized to operate in the industry.

The Drug Supply Chain Security Act creates significant roadblocks for businesses, as it takes a large-scale strategy to reach full compliance.

Blockchain technology is essentially a method of encapsulating data or information within a layered data structure so that multiple parties can trust in the accuracy of the source of information.

The Drug Supply Chain Security Act (DSCSA) requires that starting November 2017 manufacturers mark their products in human and machine-readable format with a National Drug Code (NDC).

Get detailed updates on FDA – HDA Traceability Seminar. Acquaint yourself with the requirements of new pharma laws.

Enacted in 2013, DSCSA is considered a heavy burden by many companies in the pharma supply chain.

As the Drug Supply Chain Security Act deadlines approach, serialization and aggregation coordination efforts come to the fore.

Be aware of the penalties under the Drug Supply Chain Security Act for smooth business and better delivery of pharmaceutical products.
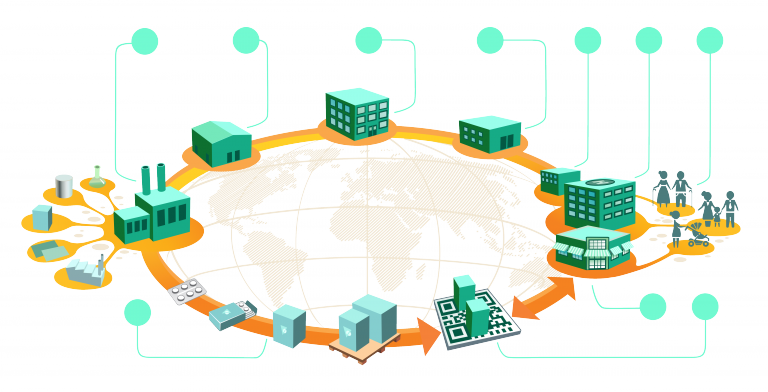
The Importance of Collaboration Across the Supply Chain is important for effectively supplying the products in time by maintaining its quality. Pharma supply chain is not an exception.
Shaping the future of traceability and serialization since 2007