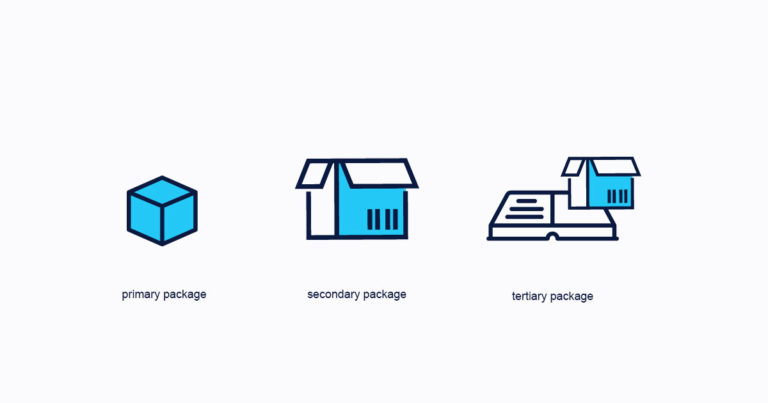
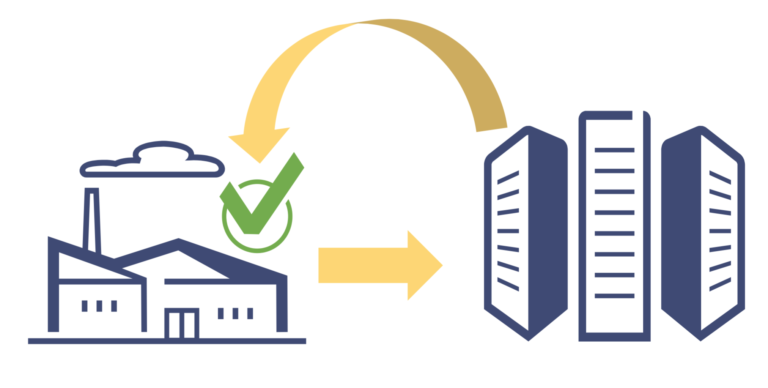
GS1 DSCSA EPCIS Version 1.2 Helps Companies Prepare for Item-Level Traceability
The Drug Supply Chain Security Act (DSCSA) requires that starting November 2017 manufacturers mark their products in human and machine-readable format with a National Drug Code (NDC).