We have less then 30 days before the DSCSA March 1st deadline. The first step in becoming compliant is creating a collaboration plan with your trading partners.
Collaboration by all parties in the supply chain has been the subject of discussion for some time. As far back as 2008, a survey of the Consumer Package Goods (CPG) industry showed that collaboration was one of the main issues concerning companies, with 80% of those surveyed saying that they were involved in at least one collaboration initiative and some involved in as many as ten.
What companies were looking for then, and are still looking for today, is:
- Cost savings resulting in improved bottom lines at a time when margins are under serious pressure
- Greater cooperation resulting from the change from an antagonistic to a cooperative stance.
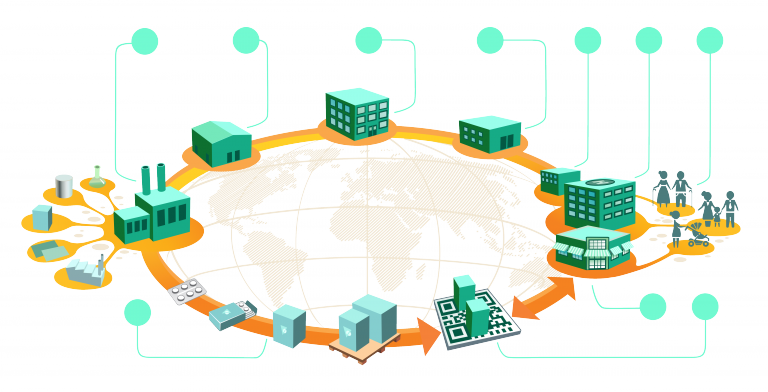
With the advent of the Drug Supply Chain Security Act (DSCSA), however, companies engaged in the manufacture, transport and sale of affected drugs have more than that to concern themselves with. DSCSA demands infallible traceability of prescription medicines and imposes on all parties the need to make sure that no failure in traceability exists. For manufacturers, wholesalers, logistics suppliers, retailers and dispensers, there is an unavoidable, unbreakable requirement to be able to track every consignment and every item on every step of its journey from laboratory bench to consumer.
No company involved in the chain can expect to meet its obligations unaided.
Timelines
There are some timelines that tell companies involved in the drug supply chain what they have to do:
- The first target date passed a year ago; from January 1 2015, trading partners have been permitted to purchase products only from authorized trading partners and must exchange transactional data on a lot batch level. Trading partners must have a system or process in place to investigate and quarantine products that are “suspect” or illegitimate, and notify the FDA and immediate trading partners.
- From November 1 2017, manufacturers must add a “product identifier” to each individual package and homogeneous case of product.
- From November 2023, the DSCSA will turn into an electronic, serialized records retention system.
Collaboration Is Not As Straightforward As It Seems
Even if all the changes that need to be made in the supply chain affected only one company, getting them right would still be difficult. There has to be a commitment to collaboration at all levels from senior management through middle managers to teams on the front line. Chinese whispers can mean that the message that started out in the boardroom is delivered to the shop floor in a completely different set of words with a different meaning. But now we are looking for collaboration not between departments but between companies, who in the past may have seen their interests as to some extent in conflict with each other.
To take the most simple example, a manufacturer may be intent on getting a new product out into the marketplace as quickly as possible, while the distributor has one eye on skinny margins and is much more interested in getting cost out of product handling and storage (an article in Supply Chain Digital suggested that the supply chain contributes between 9% and 17% of cost in the end-to-end value chain).
The DSCSA exists and, difficult or not, collaboration has to happen. With the first of our timeline points already passed and the requirement in operation, trading partners must now be looking at the November 2017 deadline for product identifiers. It is manufacturers who have to add the identifier, but if others in the chain are to find the end result satisfactory, they need to be engaging with manufacturers in a conversation about how the identifiers are to be presented.
The Elements of Supply Chain Track and Trace Technologies
Some of this is taken out of participants’ hands because the act says the product identifier is to be a standardized graphic (two-dimensional dot matrix) that carries the product’s standardized numerical identifier (SNI), lot number, and expiration date in both human-readable and machine-readable format. For those without a clear understanding of how supply chain track and trace technologies work, here is a brief introduction.
Let’s begin with the distinction between Master Data and Instance Data – or, Non-transactional Data and Transactional Data. Master Data is persistent and non-transactional; it contains a unique identifier; and it defines a business entity. “Business entity” does not just mean “company”; customer, location, employee and product are all examples of business entities that qualify for Master Data because they don’t change. The product remains the product; the location the location; the employee remains the employee.
What DSCSA requires is that Master Data applies not just within the company but right down the supply chain from beginning to end. In the past, a product may have gone through six different identifiers on its way from laboratory bench to consumer; now, there can only be one. What we are suggesting is that 2017 is not very far away and logistics companies, distributors and everyone else involved should be engaging with the manufacturers now to make sure that the Master Data identifiers allocated by the manufacturers are acceptable at all stages in the process and that the definition of the data elements is understood and accepted by all parties.
An example of what we have described as Transactional or Instance Data would be a lot number allocated to a specific batch. That batch begins its journey from the factory and ends being broken down for delivery to individual consumers. Once again, the act requires the data to remain exactly the same from one end of the chain to the other and – once again – this is the time for everyone involved in the chain to be discussing what it will contain and what it will look like. If those discussions don’t take place, the transactional data will still be created (indeed, it’s created now at the factory) and everyone will have to accept it as it is.
Free DSCSA Evaluation
With the deadline fast approaching, contact TrackTraceRx today to receive a free evaluation of your DSCSA current policy and procedures. This free consultation will allow you to have a piece of mind that you are following the correct procedures in order to meet ALL DSCSA requirements. TrackTraceRx will also provide you with a FREE Standard Operating Procedure (SOP) template which is required by the DSCSA during a FDA inspection.